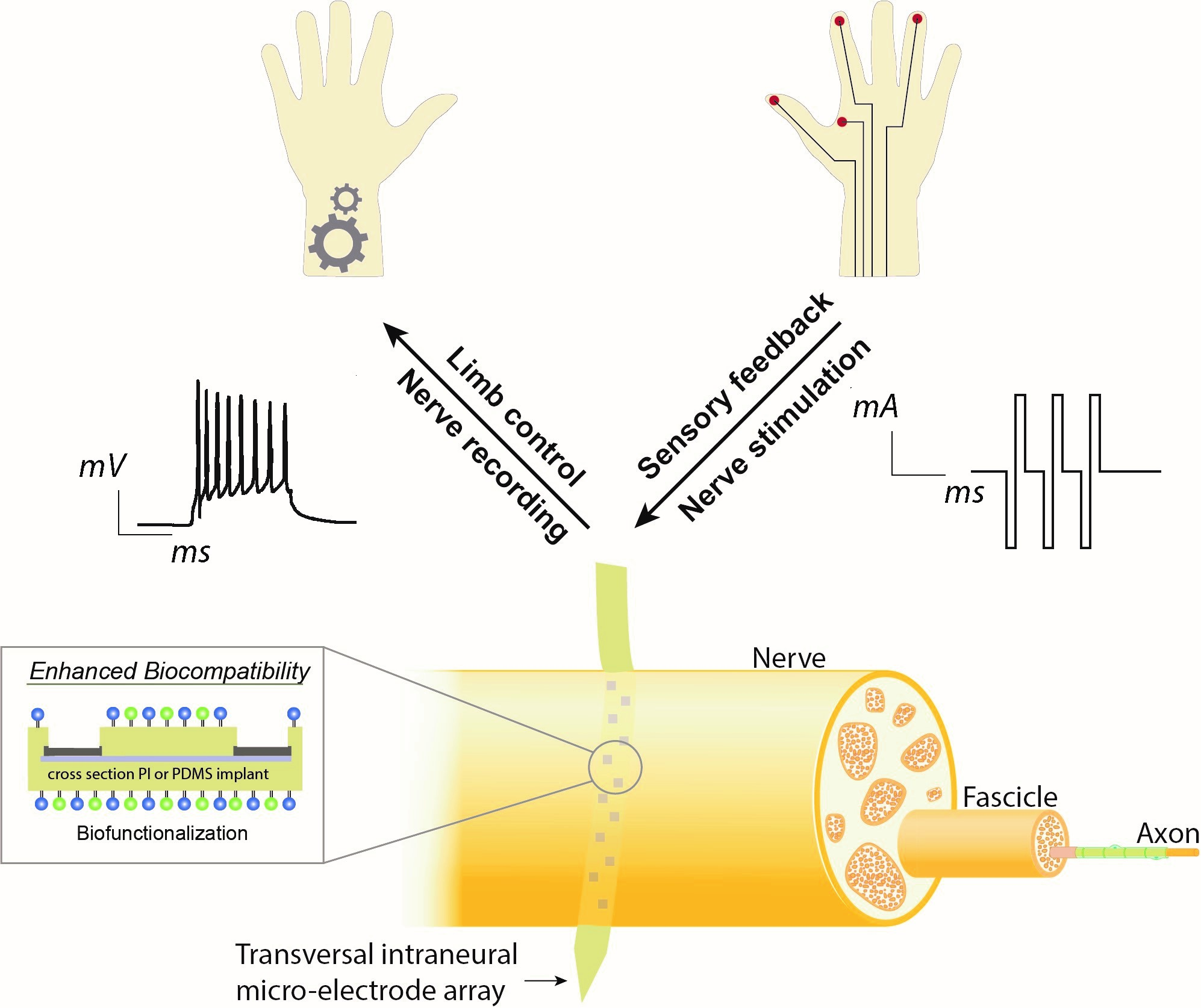
After traumatic limb loss, electrically innervated bionic limbs help patients to gain independence and improve their quality of life beyond what is possible with more conventional prostheses. However, their insufficient long-term integration with the patient’s own nervous system is a remaining barrier to broader clinical use. The astonishing potential of bionics to use finer details in nerve signaling patterns for limb control, or to provide bionic limbs with artificial sensory function, can only truly come to patient benefit when this performance remains over many years.

The BioFINE project, acronym for BioFunctional IntraNeural Electrodes, funded by the European Innovation Council within the HORIZON-EIC-2022-PATHFINDEROPEN program, aims to enhance biocompatibility of intraneural electrodes for bionic limbs as a multi-dimensional chemical-engineering challenge. New micro- and nanofabrication processes will shape implants and interconnections with individual features below one micron, making it possible to greatly reduce the impact these implants have on the surrounding tissue. Notably we will not need to reduce electrical functionality but on the contrary add more electrodes for even better interfacing of the intraneural space. To further enhance tissue integration, our implants will be dressed in bio-functional coatings that release anti-inflammatory drugs or act as biomimetic interfaces to improve cell-surface interaction. Our long-term vision is to develop the first intraneural high channel-count interface capable of harvesting/injecting signals for motor control and sensory feedback to prosthetic limbs while designed to operate over decades.
The BioFINE project started 1st of April 2023 and rely on close interaction between scientists working at University of Freiburg and Chalmers University of Technology developing the implants, University of Ferrara developing new methodology for implant biofunctionalization and the Universitat Autònoma de Barcelona analyzing the effect of these new implant concepts on the performance in the nerve. The Group of Neuroplasticity and Regeneration of the UAB, led by Prof. Xavier Navarro, will lead the validation of the new interface system in vivo. The ultimate goal is to have a combined approach to bridge the gap from electrodes to nerves and generate a long term functional peripheral nerve interface that can benefit patients long term.