MICRO-DEPOSITOS PROTEICOS BIOMIMETICOS COMO UNA PLATAFORMA DE ADMINISTRACION SOSTENIDA DE FARMACOS EN Y A TRAVES DE LA PIEL.
MICRO-DIPOSITS PROTEICS BIOMIMÈTICS COM UNA PLATAFORMA D’ADMINISTRACION SOSTINGUDA DE FÀRMACS EN I A TRAVÉS DE LA PELL.
BIOMIMETIC PROTEIN MICRO-DEPOSITS AS A PLATFORM FOR SUSTAINED DRUG DELIVERY IN AND THROUGH THE SKIN. (PERDURA).
PID2020-116174RB-I00. 1/09/2021-31/08/2024. IP A. Villaverde. 121.000 eur.
Being the major organ in the human body, skin is also an architectonically complex, stratified biological barrier. Skin limits the penetration of molecules from the environment and prevents from infection by pathogens, since its external corneal layer is formed by dead cells. Favored by skin aging that increases the fragility and vulnerability of the organ, multiple skin pathologies including skin cancers, xerosis, venous and pressure ulcers, dermatitis, eczema, diabetic feet, itch, psoriasis and a diversity of fungal, bacterial and viral infections show a significant prevalence, with an incidence expected to increase in the next decades. Some of these conditions are linked to multi-morbidity and that need to be addressed through highly complex treatments, especially in elderly people. Drug application into skin, mostly based on micro-emulsions, often poses concerns regarding drug penetrability, stability and efficacy, especially for hydrophilic agents like proteins. Also, because of the large skin surface and the simplicity of this approach, transdermal drug delivery is being largely explored, not only to treat skin conditions but also to achieve non-invasive routes to deliver remedies at the systemic level for a diversity of conditions. The importance of both in situ skin delivery and trans-dermal drug delivery pushes to develop new strategies for drug administration based on microneedles, micro implants and nanoscale drug carriers. PERDURA proposes a novel concept in drug delivery into and through skin, based on the design and testing of microscale protein depots, with defined chemical composition, biodegradable and fully compatible. Fabricated by simple technologies and in absence of xenobiotic matrices, PERDURA principles should offer not only full biocompatibility and excellent cell and tissue penetrability, but also high stability and long-term sustained delivery of protein-based drugs. The concepts supporting PERDURA are transversal enough to be developed in the harsh regulatory scenario aiming at clinical applications but also in the softer regulatory landscape of cosmetics, in which effective cosmeceuticals emerge in the market because of their increasing demand and wide social acceptance.
Publications:
https://doi.org/10.1007/s40843-021-1914-0
https://doi.org/10.3390/pharmaceutics15112632
https://doi.org/10.3390/pharmaceutics14122644
https://doi.org/10.1021/acsami.3c08643
https://doi.org/10.1016/j.biotechadv.2022.108032
https://doi.org/10.3390/pharmaceutics15041197
https://doi.org/10.1021/acsmaterialslett.3c01643
https://doi.org/10.1002/advs.202309427
https://doi.org/10.3390/nano14050435
https://doi.org/10.1021/acssuschemeng.2c06635
https://doi.org/10.1016/j.actbio.2023.09.001
https://doi.org/10.1016/j.biotechadv.2023.108103
https://doi.org/10.1016/j.biopha.2023.114976
https://doi.org/10.1016/j.ijbiomac.2023.126164
https://doi.org/10.1186/s12934-023-02081-7
https://doi.org/10.1021/acsami.4c01210
Relevant findings
16-Nov-2023
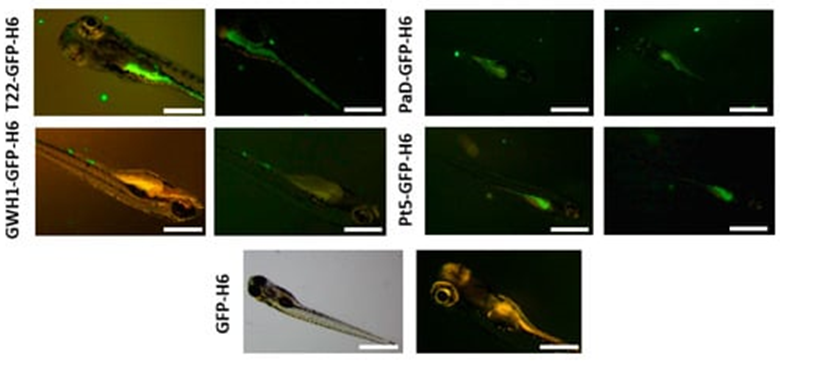
Efficient Delivery of Antimicrobial Peptides in an Innovative, Slow-Release Pharmacological Formulation (from https://doi.org/10.3390/pharmaceutics15112632)
Both nanostructure and multivalency enhance the biological activities of antimicrobial peptides (AMPs), whose mechanism of action is cooperative. In addition, the efficacy of a particular AMP should benefit from a steady concentration at the local place of action and, therefore, from a slow release after a dynamic repository. In the context of emerging multi-resistant bacterial infections and the urgent need for novel and effective antimicrobial drugs, we tested these concepts through the engineering of four AMPs into supramolecular complexes as pharmacological entities. For that purpose, GWH1, T22, Pt5, and PaD, produced as GFP or human nidogen-based His-tagged fusion proteins, were engineered as self-assembling oligomeric nanoparticles ranging from 10 to 70 nm and further packaged into nanoparticle-leaking submicron granules. Since these materials slowly release functional nanoparticles during their time-sustained unpacking, they are suitable for use as drug depots in vivo. In this context, a particular AMP version (GWH1-NIDO-H6) was selected for in vivo validation in a zebrafish model of a complex bacterial infection. The GWH1-NIDO-H6-secreting protein granules are protective in zebrafish against infection by the multi-resistant bacterium Stenotrophomonas maltophilia, proving the potential of innovative formulations based on nanostructured and slowly released recombinant AMPs in the fight against bacterial infections.
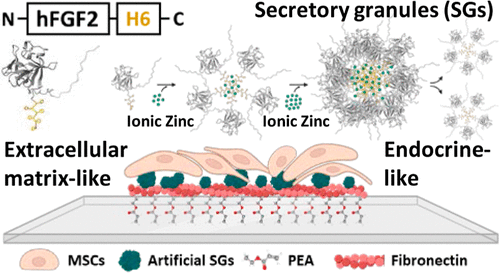
18-Jun-2024
Hybrid Micro-/Nanoprotein Platform Provides Endocrine-like and Extracellular Matrix-like Cell Delivery of Growth Factors (from https://doi.org/10.1021/acsami.4c01210)
Protein materials are versatile tools in diverse biomedical fields. Among them, artificial secretory granules (SGs), mimicking those from the endocrine system, act as mechanically stable reservoirs for the sustained release of proteins as oligomeric functional nanoparticles. Only validated in oncology, the physicochemical properties of SGs, along with their combined drug-releasing and scaffolding abilities, make them suitable as smart topographies in regenerative medicine for the prolonged delivery of growth factors (GFs). Thus, considering the need for novel, safe, and cost-effective materials to present GFs, in this study, we aimed to biofabricate a protein platform combining both endocrine-like and extracellular matrix fibronectin-derived (ECM-FN) systems. This approach is based on the sustained delivery of a nanostructured histidine-tagged version of human fibroblast growth factor 2. The GF is presented onto polymeric surfaces, interacting with FN to spontaneously generate nanonetworks that absorb and present the GF in the solid state, to modulate mesenchymal stromal cell (MSC) behavior. The results show that SGs-based topographies trigger high rates of MSCs proliferation while preventing differentiation. While this could be useful in cell therapy manufacture demanding large numbers of unspecialized MSCs, it fully validates the hybrid platform as a convenient setup for the design of biologically active hybrid surfaces and in tissue engineering for the controlled manipulation of mammalian cell growth.