Our main objective is to perform biomolecular simulations of some of the processes that, cooperatively, intervene in inflammation, in order to achieve a very detailed view of their mechanisms, and in this way to advance in the rational design of methods . and drugs that allow inflammation to be controlled with as few undesirable side effects as possible for human health.
We have divided the project into several sections, all of them sharing the characteristic of applying Theoretical Chemistry to the study of various aspects of inflammatory processes.
Lipoxygenases and Inflammation
Lipoxygenases (LOXs) form a family of lipid peroxidizing enzymes that have been implicated in a number of physiological processes and in the pathogenesis of inflammatory, hyperproliferative and neurodegenerative diseases. The LOX reaction constitutes a special type of lipid peroxidation and differs from non-enzymatic reactions in several aspects, including higher reaction rate, limited substrate selectivity, regulatory interference mechanisms, and high product specificity. Non-enzymatic lipid peroxidation converts a given substrate into a complex series of primary oxygenation products, whereas LOXs typically generate a single product isomer.
During the last few years our group has been studying the catalytic mechanism of various LOX isoforms (rabbit ALOX15, pig ALOX15, coral ALOX15b, ALOX5) with linoleic and arachidonic acids as substrate using Docking, Molecular Dynamics and QM/MM calculations. These studies have revealed the molecular origin of the exquisite regiospecificity of LOX catalysis leading to the formation of inflammatory or anti-inflammatory agents. Currently our main target is ALOX5, the most important human LOX, which catalyzes the hydroperoxidation of arachidonic acid leading to either the pro-inflammatory agents known as leukotrienes or, in combination with ALOX15, leading to the formation of lipoxins, molecules anti-inflammatory . In addition, we will focus on 5-lipoxygenase activating protein (FLAP).
Allosteric Effects of 15-LOX
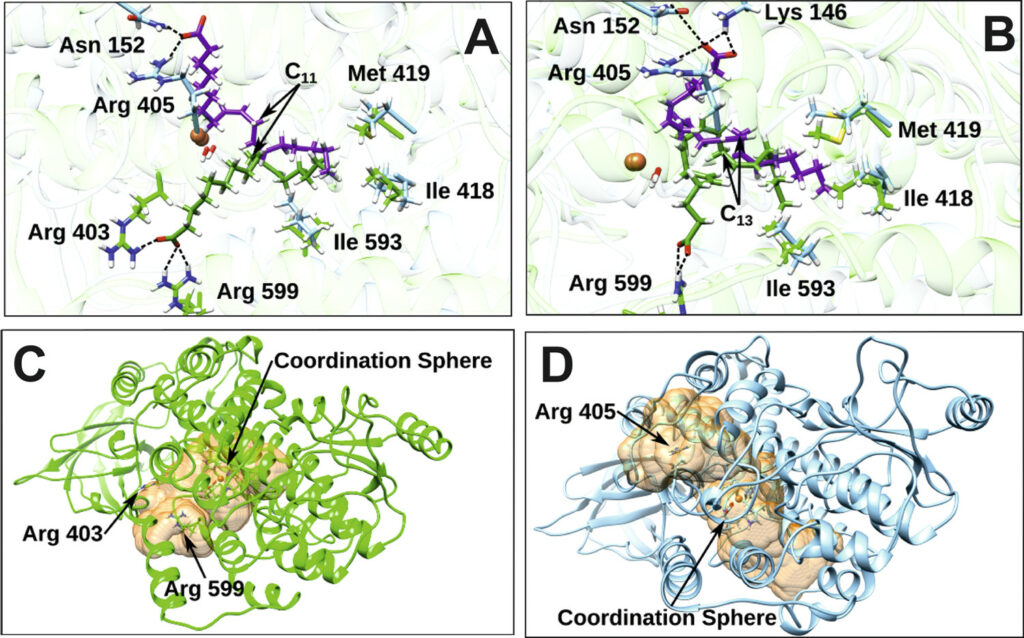
Lipoxygenases (LOXs) form a family of lipid peroxidizing enzymes that have been implicated in a number of physiological processes and in the pathogenesis of inflammatory, hyperproliferative and neurodegenerative diseases. The LOX reaction constitutes a special type of lipid peroxidation and differs from non-enzymatic reactions in several aspects, including higher reaction rate, limited substrate selectivity, regulatory interference mechanisms, and high product specificity. Non-enzymatic lipid peroxidation converts a given substrate into a complex series of primary oxygenation products, whereas LOXs typically generate a single product isomer.
During the last few years our group has been studying the catalytic mechanism of various LOX isoforms (rabbit ALOX15, pig ALOX15, coral ALOX15b, ALOX5) with linoleic and arachidonic acids as substrate using Docking, Molecular Dynamics and QM/MM calculations. These studies have revealed the molecular origin of the exquisite regiospecificity of LOX catalysis leading to the formation of inflammatory or anti-inflammatory agents. Currently our main target is ALOX5, the most important human LOX, which catalyzes the hydroperoxidation of arachidonic acid leading to either the pro-inflammatory agents known as leukotrienes or, in combination with ALOX15, leading to the formation of lipoxins, molecules anti-inflammatory . In addition, we will focus on 5-lipoxygenase activating protein (FLAP).
Cyclooxygenases and Inflammation
Prostaglandin endoperoxide H synthase (PGHS), also known as cyclooxygenase (COX), is a bifunctional enzyme with cyclooxygenase and peroxidase activities that converts arachidonic acid (AA) to prostaglandins (PG). There are two isoforms of COX, COX-1 and COX-2, and their activity is affected by aspirin treatment. While acetylated COX-1 does not produce any oxidized products, COX-2 blocks PG generation and maintains the production of 11R- and 15-HETE, metabolites with pro-resolving activity that have therapeutic implications for inflammation.
The exact mechanism that imposes stereospecificity in arachidonic acid oxygenation by COX-2 and the changes induced by aspirin treatment have not been fully elucidated. The researchers’ current goal is to use Molecular Dynamics and QM/MM calculations to better understand these processes and design new inhibitors, such as photoswitches with specific COX-2 inhibitory properties for each isomer
Design of Biocatalysts
Studies focus on understanding the mechanisms of pro-inflammatory leukotriene metabolism and designing mutated enzymes that can produce anti-inflammatory mediators. Specifically, the studies investigate the conversion of leukotriene A4 to dihydroxy leukotriene B4 by human leukotriene A4 hydrolase (LTA4H) and the synthesis of anti-inflammatory maresin compounds from epoxide intermediates using mutated LTA4H and LOX enzymes. Computational methods are used to design and test mutated enzymes, with the resulting nucleotide sequences shared with experimental collaborators for further study. The ultimate goal is to identify new pathways for the production of anti-inflammatory mediators and potentially develop new therapeutic interventions for inflammatory diseases.