Hydroperoxidation of Docosahexaenoic Acid by Human ALOX12 and pigALOX15-mini-LOX
Canyelles-Niño, M.; González-Lafont, À.; Lluch, J.M.
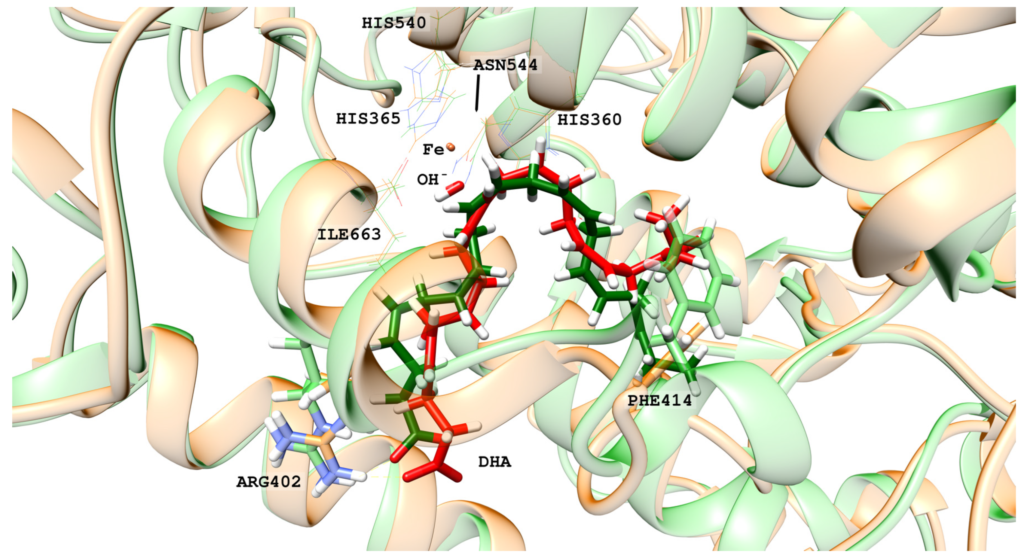
Human lipoxygenase 12 (hALOX12) catalyzes the conversion of docosahexaenoic acid (DHA) into mainly 14S-hydroperoxy-4Z,7Z,10Z,12E,16Z,19Z-docosahexaenoic acid (14S-H(p)DHA). This hydroperoxidation reaction is followed by an epoxidation and hydrolysis process that finally leads to maresin 1 (MaR1), a potent bioactive specialized pro-resolving mediator (SPM) in chronic inflammation resolution. By combining docking, molecular dynamics simulations, and quantum mechanics/molecular mechanics calculations, we have computed the potential energy profile of DHA hydroperoxidation in the active site of hALOX12. Our results describe the structural evolution of the molecular system at each step of this catalytic reaction pathway. Noteworthy, the required stereospecificity of the reaction leading to MaR1 is explained by the configurations adopted by DHA bound to hALOX12, along with the stereochemistry of the pentadienyl radical formed after the first step of the mechanism. In pig lipoxygenase 15 (pigALOX15-mini-LOX), our calculations suggest that 14S-H(p)DHA can be formed, but with a stereochemistry that is inadequate for MaR1 biosynthesis.
Special Issue “Lipid Signaling and Metabolism in Inflammation-Associated Diseases” by Prof. Dr. Hartmut Kühn
When the human body is challenged by pathogens, cell damage or irritants, a complex counteracting response of the immune system is initiated. This response is aimed at eliminating the inflammatory stimuli and at reestablishing tissue homeostasis. Although the inflammatory response is beneficial for the entire body, it involves locally destructive processes leading to cell injury and tissue damage. During the phase of inflammatory resolution (healing phase) the inflamed tissue is cleaned up and the original tissue structure is reestablished. In principle, inflammation can affect all organs, and thus can impact organ-specific functions. However, despite tissue-specific differences, the basic mechanisms are always similar. In most cases, inflammation starts as an acute process which either heals completely (restitution ad integrum) or turns into chronic inflammation when the healing process is incomplete. A key event in acute inflammation is the local activation of immune cells, particularly of neutrophils and pro-inflammatory M1 macrophages. Lymphocytes (B- and T- cells) are rare in acutely inflamed tissue, but occur more frequently in chronic inflammation. Inflammatory cells are attracted by pro-inflammatory signals produced in inflamed tissue. They leave the vasculature and actively migrate towards the center of inflammation following the gradient of pro-inflammatory mediators. Acute inflammation and inflammatory resolution are characterized by specific profiles of lipid mediators, which are biosynthesized by different cell types. It is the major aim of this Special Issue to summarize our current knowledge on lipid signaling and lipid metabolism in all types of inflammation-associated diseases, which includes the biosynthesis and modes of action of pro- and anti-inflammatory lipid mediators.