Àngels González Lafont received a doctorate in Chemical Sciences from the Universitat Autònoma de Barcelona (UAB) in 1989 and she became university professor at the UAB in 1992. Full Professor of Physical Chemistry at the UAB since 2011. Director of 20 PhD theses (+1 in progress), including one with an Industrial Doctorate in collaboration with a biotechnology company. Publication of 169 scientific articles (+4 in the field of teaching).
My scientific career began in the field of Atmospheric Chemistry. I used variational transition state theory (VTST), including tunneling corrections, to calculate the rate constants of significant atmospheric degradation chemical processes initiated by the OH radical. In 2003, my research evolved towards biomolecular simulations of enzyme catalysis. I started using classical Molecular Dynamics simulations using a full model of biomolecules with thousands of atoms and water molecules to do QM/MM calculations to study the potential energy surfaces of different catalytic mechanisms which allowed me to have an extensive knowledge of the behavior and reactivity of cyclooxygenases and lipoxygenases.
My efforts are now focused on using all my theoretical knowledge of lipoxygenases (ALOX5, ALOX15 and ALOX12) and COX-2 together with my expertise in computational methodologies to model biomolecular systems to develop innovative Molecular Biomedicine approaches to block the pro-inflammatory action of these enzymes and activate their function to resolve the inflammatory processes that are the underlying cause of most cancers. The rational design of new COX-2 and ALOX5 inhibitors (mostly photoswitchable drugs), the biocatalytic design of MaR1 synthesis and the design of agonists is the focus of my current research.
Latest Publications
Protein Science
First-principles simulations of the fluorescence modulation of a COX-2-specific fluorogenic probe upon protein dimerization for cancer discrimination
ACS Catalysis
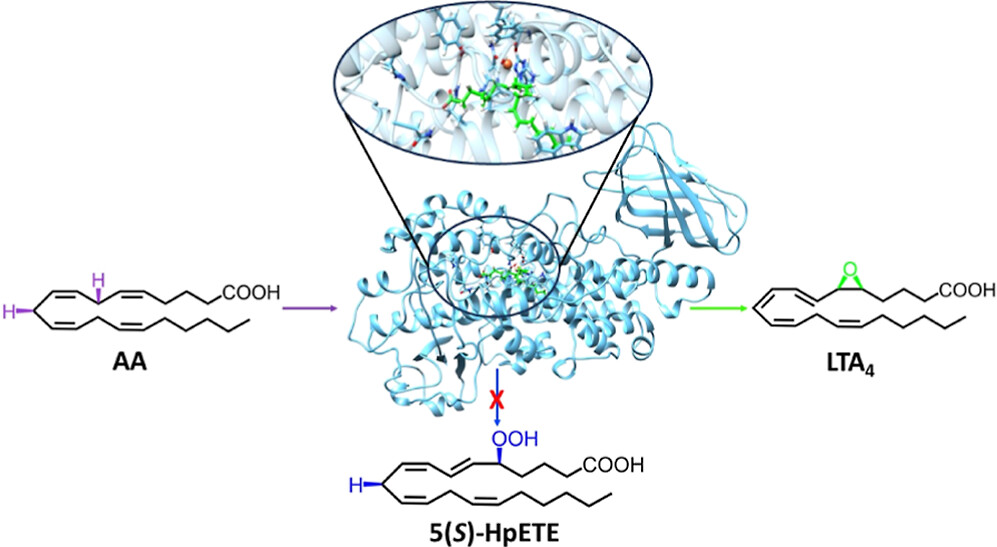
Theoretical Study of the Arachidonic Acid Conversion into Leukotriene A4 Catalyzed by Human 5-Lipoxygenase: Hydroperoxidation and Epoxidation Mechanisms and Arachidonic Acid Active Site Access
Molecules
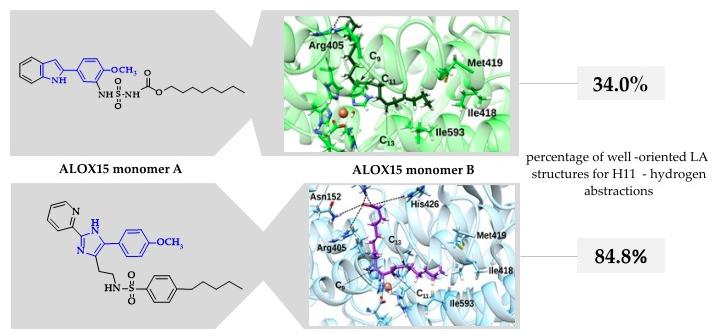
Different Structures—Similar Effect: Do Substituted 5-(4-Methoxyphenyl)-1H-indoles and 5-(4-Methoxyphenyl)-1H-imidazoles Represent a Common Pharmacophore for Substrate Selective Inhibition of Linoleate Oxygenase Activity of ALOX15?