2025
Exploring allosteric properties of mammalian ALOX15: octyl (N-(4-(benzofuran-2-yl)-2-methoxyphenyl)sulfamoyl)- and octyl (N-(4-(1H-indol-2-yl)-2-methoxyphenyl)sulfamoyl)carbamates as ALOX inhibitors
Viktor Gavrilyuk, Alejandro Cruz, Vladislav Aksenov, Danila Nurgaliev, Alexander Zhuravlev, Alexey Golovanov, José M. Lluch, Hartmut Kuhn, Igor Ivanov i Àngels González-Lafont
RSC Adv., 2025, 15, 32284
First-principles simulations of the fluorescence modulation of a COX-2-specific fluorogenic probe upon protein dimerization for cancer discrimination
Álex Pérez-Sánchez, Carles Curutchet, Àngels González-Lafont i José M. Lluch
Protein Science 2025;34(1):e70001.
2024
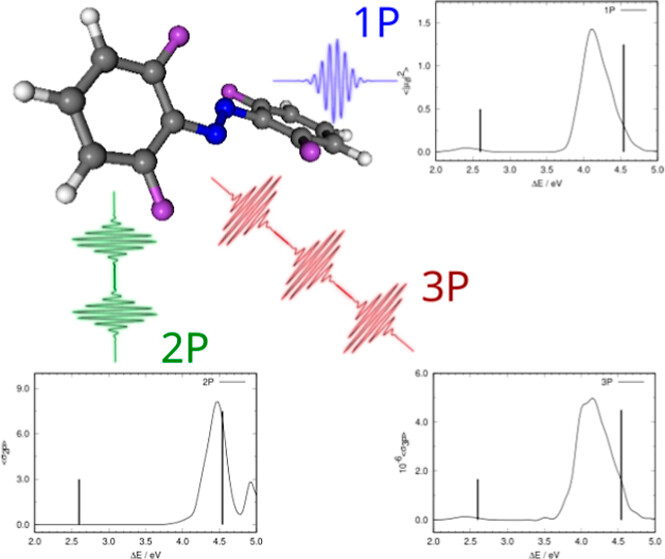
Effect of Leaving Centrosymmetric Character on Spectral Properties in Mono-, Bi-, and Triphotonic Absorption Spectroscopies
Ricard Gelabert, Miquel Moreno i José M. Lluch
ACS Omega 2024, 9, 40, 41968–41977
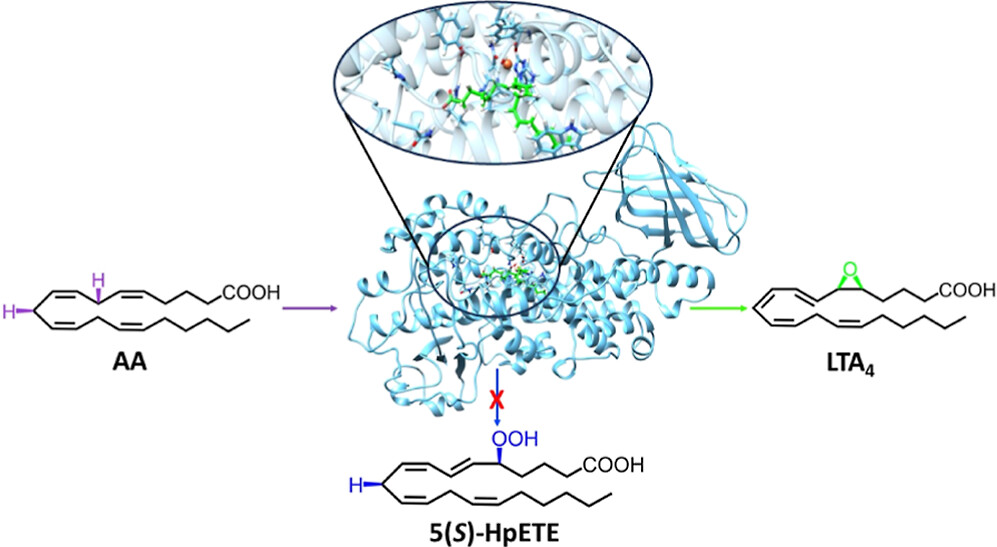
Theoretical Study of the Arachidonic Acid Conversion into Leukotriene A4 Catalyzed by Human 5-Lipoxygenase: Hydroperoxidation and Epoxidation Mechanisms and Arachidonic Acid Active Site Access
Alejandro Cruz, Àngels González-Lafont, José Maria Lluch
ACS Catal. 2024, 14, 2, 637–656
2023
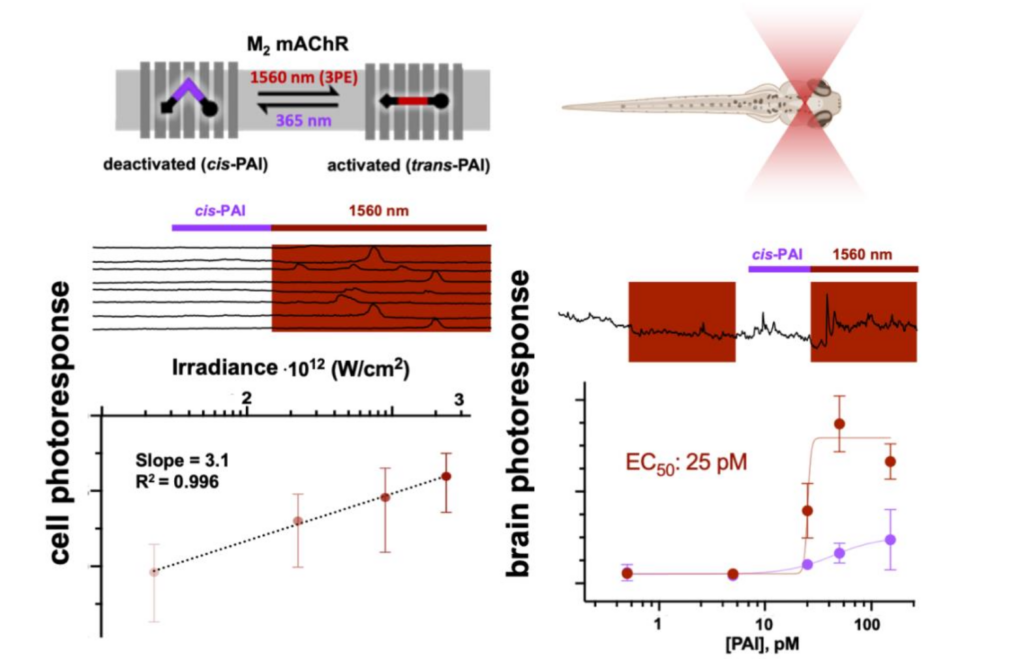
Three-Photon Infrared Stimulation of Endogenous Neuroreceptors in Vivo
Rosalba Sortino, Marina Cunquero, Gustavo Castro-Olvera, Ricard Gelabert, Miquel Moreno, Fabio Riefolo, Carlo Matera, Noèlia Fernàndez-Castillo, Luca Agnetta, Michael Decker, José Maria Lluch, Jordi Hernando, Pablo Loza-Alvarez, Pau Gorostiza
Angew. Chem. Int. Ed. 2023
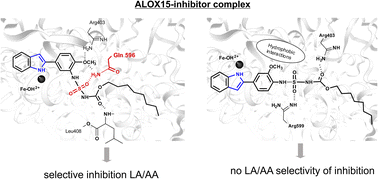
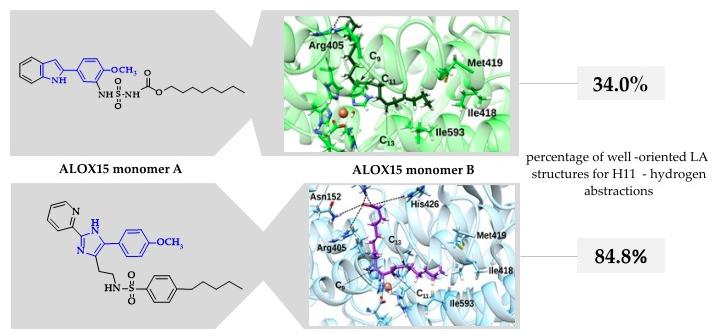
Different Structures—Similar Effect: Do Substituted 5-(4-Methoxyphenyl)-1H-indoles and 5-(4-Methoxyphenyl)-1H-imidazoles Represent a Common Pharmacophore for Substrate Selective Inhibition of Linoleate Oxygenase Activity of ALOX15?
Zhuravlev, A.; Cruz, A.; Chen, X.; Aksenov, V.; Golovanov, A.; Lluch J. M.; Kuhn, H.; González-Lafont, À. Ivanov I.
Molecules 2023, 28, 5418
Resum
Mammalian 15-lipoxygenases (ALOX15) are lipid peroxidizing enzymes that exhibit variable functionality in different cancer and inflammation models. The pathophysiological role of linoleic acid- and arachidonic acid-derived ALOX15 metabolites rendered this enzyme a target for pharmacological research. Several indole and imidazole derivatives inhibit the catalytic activity of rabbit ALOX15 in a substrate-specific manner, but the molecular basis for this allosteric inhibition remains unclear. Here, we attempt to define a common pharmacophore, which is critical for this allosteric inhibition. We found that substituted imidazoles induce weaker inhibitory effects when compared with the indole derivatives. In silico docking studies and molecular dynamics simulations using a dimeric allosteric enzyme model, in which the inhibitor occupies the substrate-binding pocket of one monomer, whereas the substrate fatty acid is bound at the catalytic center of another monomer within the ALOX15 dimer, indicated that chemical modification of the core pharmacophore alters the enzyme–inhibitor interactions, inducing a reduced inhibitory potency. In our dimeric ALOX15 model, the structural differences induced by inhibitor binding are translated to the hydrophobic dimerization cluster and affect the structures of enzyme–substrate complexes. These data are of particular importance since substrate-specific inhibition may contribute to elucidation of the putative roles of ALOX15 metabolites derived from different polyunsaturated fatty acids in mammalian pathophysiology.
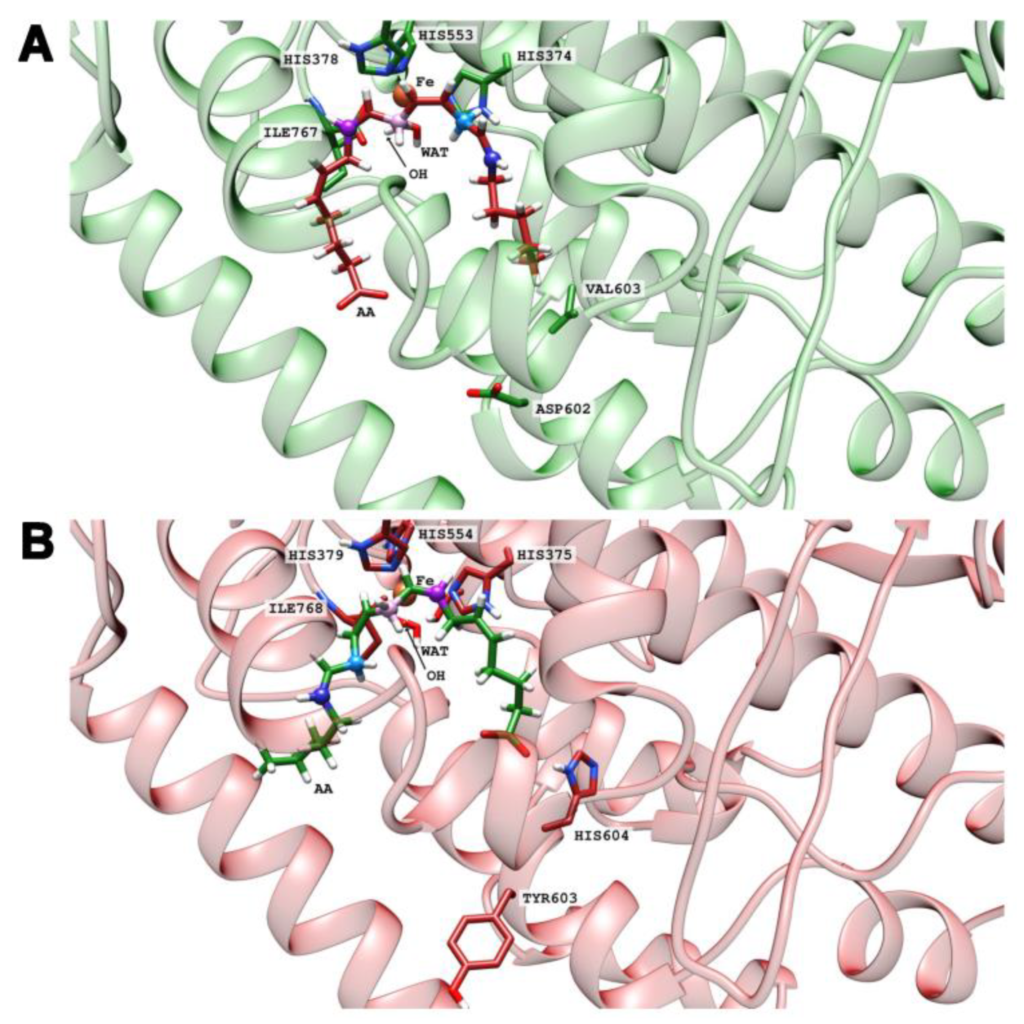
Functional Characterization of Mouse and Human Arachidonic Acid Lipoxygenase 15B (ALOX15B) Orthologs and of Their Mutants Exhibiting Humanized and Murinized Reaction Specificities
Kakularam, K. R.; Canyelles-Niño, M.; Chen, X.; Lluch J. M.; González-Lafont, À. González-Lafont; Kuhn, H.
Int. J. Mol. Sci. 2023, 24(12), 10046
Resum
The arachidonic acid lipoxygenase 15B (ALOX15B) orthologs of men and mice form different reaction products when arachidonic acid is used as the substrate. Tyr603Asp+His604Val double mutation in mouse arachidonic acid lipoxygenase 15b humanized the product pattern and an inverse mutagenesis strategy murinized the specificity of the human enzyme. As the mechanistic basis for these functional differences, an inverse substrate binding at the active site of the enzymes has been suggested, but experimental proof for this hypothesis is still pending. Here we expressed wildtype mouse and human arachidonic acid lipoxygenase 15B orthologs as well as their humanized and murinized double mutants as recombinant proteins and analyzed the product patterns of these enzymes with different polyenoic fatty acids. In addition, in silico substrate docking studies and molecular dynamics simulation were performed to explore the mechanistic basis for the distinct reaction specificities of the different enzyme variants. Wildtype human arachidonic acid lipoxygenase 15B converted arachidonic acid and eicosapentaenoic acid to their 15-hydroperoxy derivatives but the Asp602Tyr+Val603His exchange murinized the product pattern. The inverse mutagenesis strategy in mouse arachidonic acid lipoxygenase 15b (Tyr603Asp+His604Val exchange) humanized the product pattern with these substrates, but the situation was different with docosahexaenoic acid. Here, Tyr603Asp+His604Val substitution in mouse arachidonic acid lipoxygenase 15b also humanized the specificity but the inverse mutagenesis (Asp602Tyr+Val603His) did not murinize the human enzyme. With linoleic acid Tyr603Asp+His604Val substitution in mouse arachidonic acid lipoxygenase 15b humanized the product pattern but the inverse mutagenesis in human arachidonic acid lipoxygenase 15B induced racemic product formation. Amino acid exchanges at critical positions of human and mouse arachidonic acid lipoxygenase 15B orthologs humanized/murinized the product pattern with C20 fatty acids, but this was not the case with fatty acid substrates of different chain lengths. Asp602Tyr+Val603His exchange murinized the product pattern of human arachidonic acid lipoxygenase 15B with arachidonic acid, eicosapentaenoic acid, and docosahexaenoic acid. An inverse mutagenesis strategy on mouse arachidonic acid lipoxygenase 15b (Tyr603Asp+His604Val exchange) did humanize the reaction products with arachidonic acid and eicosapentaenoic acid, but not with docosahexaenoic acid.
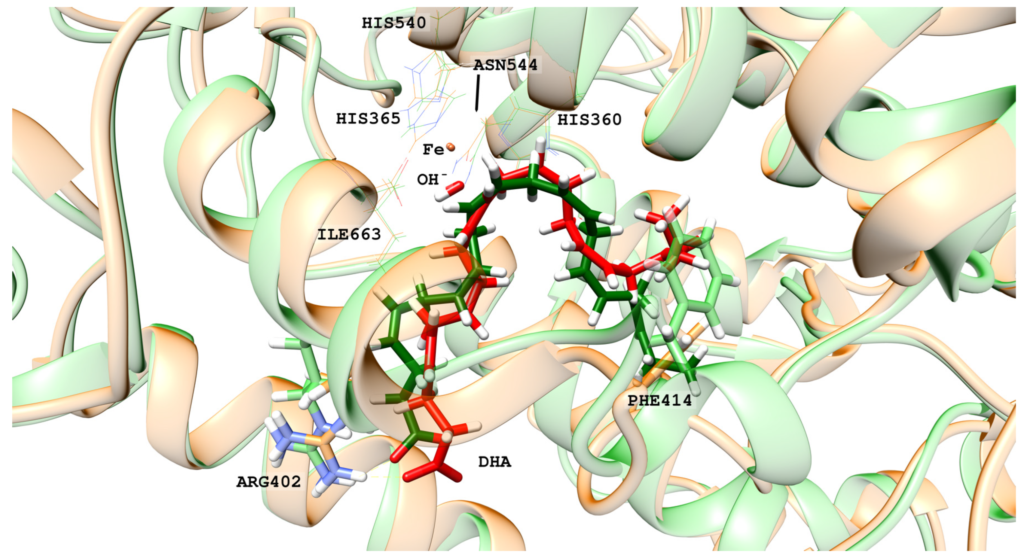
Hydroperoxidation of Docosahexaenoic Acid by Human ALOX12 and pigALOX15-mini-LOX
Canyelles-Niño, M.; González-Lafont, À.; Lluch, J.M.
Int. J. Mol. Sci. 2023, 24(7), 6064
Resum
Human lipoxygenase 12 (hALOX12) catalyzes the conversion of docosahexaenoic acid (DHA) into mainly 14S-hydroperoxy-4Z,7Z,10Z,12E,16Z,19Z-docosahexaenoic acid (14S-H(p)DHA). This hydroperoxidation reaction is followed by an epoxidation and hydrolysis process that finally leads to maresin 1 (MaR1), a potent bioactive specialized pro-resolving mediator (SPM) in chronic inflammation resolution. By combining docking, molecular dynamics simulations, and quantum mechanics/molecular mechanics calculations, we have computed the potential energy profile of DHA hydroperoxidation in the active site of hALOX12. Our results describe the structural evolution of the molecular system at each step of this catalytic reaction pathway. Noteworthy, the required stereospecificity of the reaction leading to MaR1 is explained by the configurations adopted by DHA bound to hALOX12, along with the stereochemistry of the pentadienyl radical formed after the first step of the mechanism. In pig lipoxygenase 15 (pigALOX15-mini-LOX), our calculations suggest that 14S-H(p)DHA can be formed, but with a stereochemistry that is inadequate for MaR1 biosynthesis.
Predicting the Electronic Absorption Band Shape of Azobenzene Photoswitches
Moreno, M.; Lluch, J.M.; Gelabert, R.
Int. J. Mol. Sci. 2023, 24(1), 25
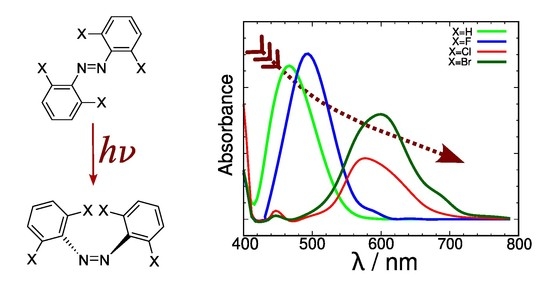
Resum
Simulations based on molecular dynamics coupled to excitation energy calculations were used to generate simulated absorption spectra for a family of halide derivatives of azobenzene, a family of photoswitch molecules with a weak absorption band around 400–600 nm and potential uses in living tissue. This is a case where using the conventional approach in theoretical spectroscopy (estimation of absorption maxima based on the vertical transition from the potential energy minimum on the ground electronic state) does not provide valid results that explain how the observed band shape extends towards the low energy region of the spectrum. The method affords a reasonable description of the main features of the low-energy UV-Vis spectra of these compounds. A bathochromic trend was detected linked to the size of the halide atom. Analysis of the excitation reveals a correlation between the energy of the molecular orbital where excitation starts and the energy of the highest occupied atomic orbital of the free halide atom. This was put to the test with a new brominated compound with good results. The energy level of the highest occupied orbital on the free halide was identified as a key factor that strongly affects the energy gap in the photoswitch. This opens the way for the design of bathochromically shifted variants of the photoswitch with possible applications.
2022
On the Computational Design of Azobenzene-Based Multi-State Photoswitches
Moreno, M.; Lluch, J.M.; Gelabert, R.
Int. J. Mol. Sci. 2022, 23(15), 8690
Resum
In order to theoretically design multi-state photoswitches with specific properties, an exhaustive computational study is first carried out for an azobenzene dimer that has been recently synthesized and experimentally studied. This study allows for a full comprehension of the factors that govern the photoactivated isomerization processes of these molecules so to provide a conceptual/computational protocol that can be applied to generic multi-state photoswitches. From this knowledge a new dimer with a similar chemical design is designed and also fully characterized. Our theoretical calculations predict that the new dimer proposed is one step further in the quest for a double photoswitch, where the four metastable isomers could be selectively interconverted through the use of different irradiation sequences.
On the Computational Molecular Insights into the Regulation of 3-Phosphoinositide-Dependent Protein Kinase 1: Modeling the Interaction between the Kinase and the Pleckstrin Homology Domains
Garcia-Viloca, M.; Bayascas, J.M.; Lluch, J.M.; González-Lafont, À.
ACS Omega 2022, 7, 29, 25186–25199
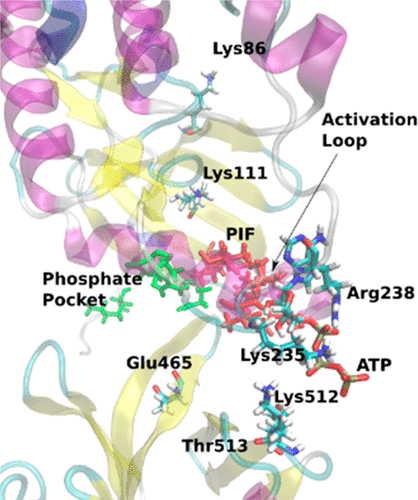
Resum
The 3-phosphoinositide-dependent protein kinase 1 (PDK1) K465E mutant kinase can still activate protein kinase B (PKB) at the membrane in a phosphatidylinositol-3,4,5-trisphosphate (PIP3, PtdIns(3,4,5)P3) independent manner. To understand this new PDK1 regulatory mechanism, docking and molecular dynamics calculations were performed for the first time to simulate the wild-type kinase domain–pleckstrin homology (PH) domain complex with PH-in and PH-out conformations. These simulations were then compared to the PH-in model of the KD–PH(mutant K465E) PDK1 complex. Additionally, three KD–PH complexes were simulated, including a substrate analogue bound to a hydrophobic pocket (denominated the PIF-pocket) substrate-docking site. We find that only the PH-out conformation, with the PH domain well-oriented to interact with the cellular membrane, is active for wild-type PDK1. In contrast, the active conformation of the PDK1 K465E mutant is PH-in, being ATP-stable at the active site while the PIF-pocket is more accessible to the peptide substrate. We corroborate that both the docking-site binding and the catalytic activity are in fact enhanced in knock-in mouse samples expressing the PDK1 K465E protein, enabling the phosphorylation of PKB in the absence of PIP3 binding.
Theoretical Characterization of the Step-by-Step Mechanism of Conversion of Leukotriene A4 to Leukotriene B4 Catalysed by the Enzyme Leukotriene A4 Hydrolase
Canyelles-Niño, M.; González-Lafont, À.; Lluch, J.M.
Int. J. Mol. Sci. 2022, 23(6), 3140
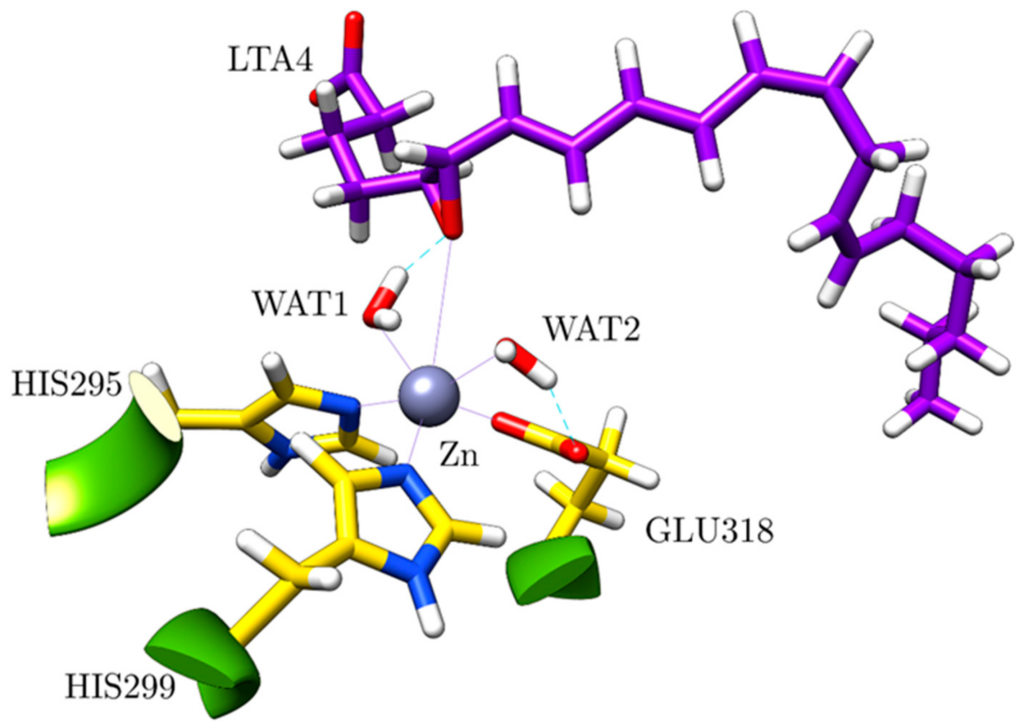
Resum
LTA4H is a bifunctional zinc metalloenzyme that converts leukotriene A4 (LTA4) into leukotriene B4 (LTB4), one of the most potent chemotactic agents involved in acute and chronic inflammatory diseases. In this reaction, LTA4H acts as an epoxide hydrolase with a unique and fascinating mechanism, which includes the stereoselective attachment of one water molecule to the carbon backbone of LTA4 several methylene units away from the epoxide moiety. By combining Molecular Dynamics simulations and Quantum Mechanics/Molecular Mechanics calculations, we obtained a very detailed molecular picture of the different consecutive steps of that mechanism. By means of a rather unusual 1,7-nucleophilic substitution through a clear SN1 mechanism, the epoxide opens and the triene moiety of the substrate twists in such a way that the bond C6-C7 adopts its cis (Z) configuration, thus exposing the R face of C12 to the addition of a water molecule hydrogen-bonded to ASP375. Thus, the two stereochemical features that are required for the bioactivity of LTB4 appear to be closely related. The noncovalent π-π stacking interactions between the triene moiety and two tyrosines (TYR267 and, especially, TYR378) that wrap the triene system along the whole reaction explain the preference for the cis configuration inside LTA4H.
N-Substituted 5-(1H-Indol-2-yl)-2-methoxyanilines Are Allosteric Inhibitors of the Linoleate Oxygenase Activity of Selected Mammalian ALOX15 Orthologs: Mechanism of Action
Golovanov, A.; Zhuravlev, A.; Cruz, A.; Aksenov, V.; Shafiullina, R.; Kakularam, K.R.; Lluch, J.M.; Kuhn, H.; González-Lafont, À; Ivanov, I.
J. Med. Chem. 2022, 65, 3, 1979–1995
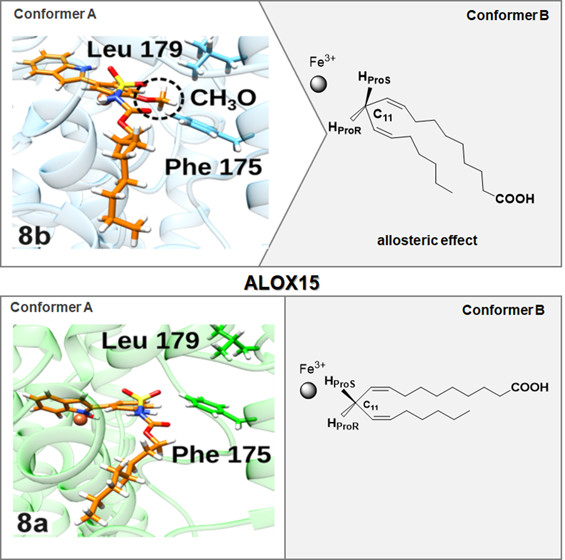
Resum
Here, we describe the first systematic study on the mechanism of substrate-selective inhibition of mammalian ALOX15 orthologs. For this purpose, we prepared a series of N-substituted 5-(1H-indol-2-yl)anilines and found that (N-(5-(1H-indol-2-yl)-2-methoxyphenyl)sulfamoyl)carbamates and their monofluorinated analogues are potent and selective inhibitors of the linoleate oxygenase activity of rabbit and human ALOX15. Introduction of a 2-methoxyaniline moiety into the core pharmacophore plays a crucial role in substrate-selective inhibition of ALOX15-catalyzed oxygenation of linoleic acid at submicromolar concentrations without affecting arachidonic acid oxygenation. Steady-state kinetics, mutagenesis studies, and molecular dynamics (MD) simulations suggested an allosteric mechanism of action. Using a dimer model of ALOX15, our MD simulations suggest that the binding of the inhibitor at the active site of one monomer induces conformational alterations in the other monomer so that the formation of a productive enzyme–linoleic acid complex is energetically compromised.
The role of acetylated cyclooxygenase-2 in the biosynthesis of resolvin precursors derived from eicosapentaenoic acid
Cebrián-Prats, A; Pinto, A.; González-Lafont, À.; Fernandes, P.A.; Lluch, J.M.
Org. Biomol. Chem., 2022, 20, 1260-1274
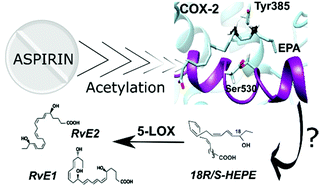
Resum
Specialized pro-resolving lipid mediators (SPMs) are natural bioactive agents actively involved in inflammation resolution. SPMs act when uncontrolled inflammatory processes are developed, for instance, in patients of COVID-19 or other diseases. The so-called resolution pharmacology aims at developing new treatments based on the use of SPMs as agonists, which promote inflammation resolution without unwanted side effects. It has been shown that the biosynthesis of SPMs called eicosapentaenoic acid (EPA)-derived E-series resolvins is initiated by aspirin-acetylated COX-2 from EPA, leading to 18-hydroperoxy-eicosapentaenoic acid (18-HpEPE). However, there are many open questions concerning the intriguing role of aspirin in the molecular mechanism of resolvin formation. Our MD simulations, combined with QM/MM calculations, show that the potential energy barriers for the H16-abstraction from EPA, required for forming 18-HpEPE, are higher than for the H13-abstraction, thus explaining why 18-HpEPE is a marginal product of COX-2 catalysis. By contrast, in the aspirin-acetylated COX-2/EPA complex, the H16proS-abstraction energy barriers are somewhat lower than the H13proS energy barriers and much smaller than the H16-transfer barriers in the wild type COX-2/EPA system. Those results agree with the experimental observation that aspirin favours the synthesis of several SPMs known as aspirin-triggered resolvins. In the following step of the catalytic mechanism, the calculated O2 addition to C18 is preferred versus the addition to C14 which also agrees with 18R-HEPE and 18S-HEPE being the main products from EPA in aspirin-acetylated COX-2.