Theoretical Study of the Arachidonic Acid Conversion into Leukotriene A4 Catalyzed by Human 5-Lipoxygenase: Hydroperoxidation and Epoxidation Mechanisms and Arachidonic Acid Active Site Access
Alejandro Cruz, Àngels González-Lafont, José M. Lluch
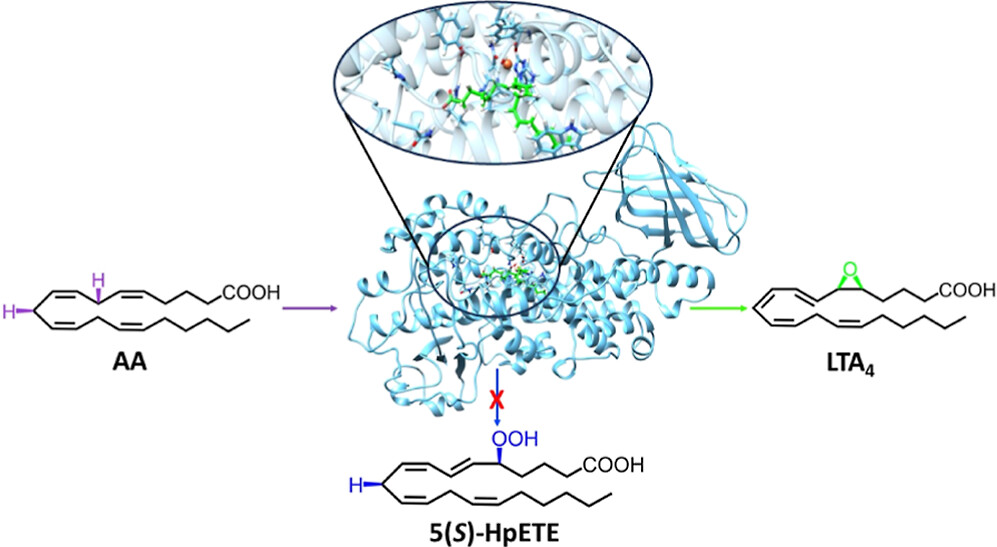
Inflammation is at the base of many different diseases. Leukotrienes (LTs) are pro-inflammatory mediators derived from arachidonic acid (AA), which play significant roles in acute inflammation. Lipoxins are specialized pro-resolving mediators (SPMs), also formed from AA, that promote the resolution of acute inflammation. However, if resolution fails, chronic inflammatory processes might develop. The enzyme human-5-lipoxygenase (5-LOX) catalyzes the biosynthesis of leukotriene named LTA4 but also intervenes in the formation of the lipoxin LXA4. These two biological functions have made the 5-LOX isoform a current target for pharmaceutical investigations in several inflammatory-based diseases searching for inhibitors that block the leukotriene reaction pathway but not lipoxin’s formation. However, the development of those selective inhibitors has been hampered by the lack of a crystal structure of human 5-LOX. In this work, we have built a complete solvated model of the human-5-LOX: AA Michaelis complex using, as initial coordinates, the human 5-LOX structure from the AlphaFold protein structure database. We aim to analyze at the molecular level the overall catalytic mechanism of 5-LOX that first converts AA into 5(S)-HpETE through a hydroperoxidation reaction and, second, transforms this hydroperoxide into LTA4 following an epoxidation process. Methodologically, we have performed molecular dynamics simulations and quantum mechanics/molecular mechanics calculations. The free energy profiles for AA entrance into the 5-LOX’s binding cavity have been calculated by steered molecular dynamics. This detailed molecular information can explain human-5-LOX’s in vitro activity (without the presence of the membrane-embedded 5-lipoxygenase-activating protein) and help to design selective inhibitors favoring inflammation resolution.